Magnesium is a lightweight, machinable, corrosion-resistant, high-strength metal that can be alloyed with small quantities of other metals, such as aluminum, manganese, zinc, and zirconium, to improve the properties.
However, these properties make magnesium and magnesium alloys tricky to weld. While thicker magnesium plates are commonly joined using TIG welding, magnesium brazing is often preferred for thinner metals.

In this article, we will explain how to braze magnesium, the advantages of brazed joints, and the differences between welding and brazing magnesium.
Fundamentals of Magnesium Brazing
Magnesium is often used for its low density, to save on weight in applications like aerospace structures. Also, the metal properties minimize inertial forces with high-speed moving parts.
You should know that brazing magnesium is quite similar to brazing aluminum. However, since brazing can use various heat sources, magnesium alloy can be joined using manual torch brazing, furnace brazing, and dip brazing.
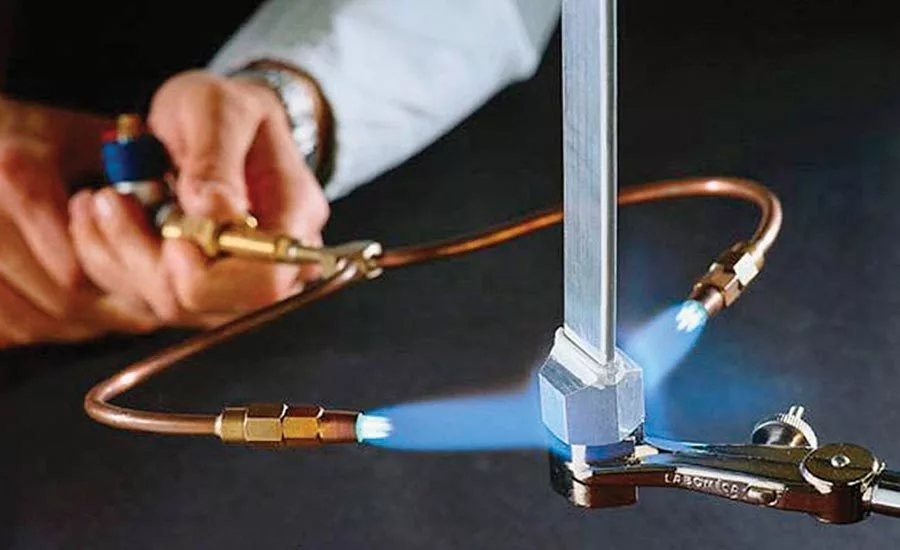
Furnace and torch brazing is generally limited to M1A alloys. Meanwhile, flux dip brazing can be used on AX10, AX31B, K1A, M1A, and ZE10A magnesium alloys.
Since magnesium can have beneficial mechanical properties at 450 °C (840 °F), brazing is a common choice. The brazing temperature is high enough to melt brazing filler metal but controlled not to reach the melting point of the base metal.
Overheating magnesium can affect the mechanical properties and lead to annealed conditions. That’s the main similarity between magnesium alloys and aluminum alloys.
Brazing Filler Metal for Magnesium alloys
Fundamentally, there are only several commercially available filler metals for brazing magnesium: BMg-1, BMg-2a and MC3 alloy.
These are all standard brazing filler metals, but they cannot be used for brazing-magnesium matrix composites due to their low solidus limitation.
In addition, high brazing temperature adversely affects the composites’ microstructure.
The brazing temperature of brazing filler metals should be as low as possible due to the low recrystallization temperature of the matrix. That’s why occasionally soldering filler metal is used.

Joining cast composites based on magnesium alloy ZK51A requires recommended brazing temperature of 480-520 °C (896-968 °F). That’s why few systems such as Al-Mg-Cu, Mg-Al-Ca, Mg-Li-Al-Zn, and Mg-Al-Zn-Ca were developed to improve mechanical properties.
Low-temperature filler metals for furnace brazing magnesium matrix composites at 450–520 °C (842–968 °F) are now widely used. These filler metals provide at least 175 MPa (25 ksi) shear and tensile strengths in the brazed joints.
Also for joining wrought, work-hardened and tempered magnesium alloys, brazing filler metals have a low-temperature range of 490–520 °C (914–968 °F). In addition, filler metals designed for brazing extruded or rolled magnesium matrix composites should have as low as possible a brazing temperature.
Magnesium Alloy Brazing Fluxes
Fundamentally, fluxes remove oxides and other contaminants in order to create solid, high-quality brazed joints.
When brazing magnesium, chloride-based fluxes are suitable, similar to those used in gas welding.
However, keep in mind that special flux is required for furnace brazing. Meanwhile, fluxes with a water or alcohol base are unsuitable for furnace brazing.

Furnace and flux pots are equipped with automatic controls to stabilize the temperature within ± 5ºF (2.7ºC) and avoid overheating the base metal.
Surface Preparation and Cleaning
Keep in mind that all brazed parts must be thoroughly cleaned prior to brazing. Substances such as oil, grease, dirt, and surface films such as chromates and oxides can affect the strength of the brazed joints and prevent capillary attraction which is crucial to brazing.
Chemical cleaning can be accomplished by vapor degreasing, alkaline cleaning, or acid cleaning. You can perform mechanical cleaning by sanding with an aluminum oxide cloth.

However, there are special precautions. Pre-cleaning and post-cleaning acids used in magnesium welding and brazing are highly toxic and corrosive. That’s why it is recommended to wear goggles, rubber gloves, and rubber aprons when dealing with acids and solutions.
Always protect yourself from fumes and mists by using a respiratory system or ventilation. When spilled on the body or clothing, wash immediately with large quantities of cold water, and seek medical attention.
You should never pour water into acid when preparing solutions. On the contrary, pour acid into water, and mix slowly to avoid spilling.
Besides cleaning, you should provide suitable clearance between parts. The suggested clearance from 0.004 to 0.010 in. (0.102 to 0.254 mm) is enough to encourage capillary attraction.
Post-Braze Cleaning
Even though brazing requires significantly less post-cleaning, you will still have to clean the remaining flux.
The flux residues are hygroscopic and will cause corrosion and loss of beneficial properties. Therefore, you should post-braze clean the parts in the same manner as for gas welded components.
Brazing Magnesium Types and Tips
- Torch brazing: The torch brazing of magnesium and magnesium alloys utilizes the same equipment as gas welding. You should use neutral oxyacetylene or a natural gas-air flame because of its soft flame and less danger of overheating. If you use a filler rod, the flame is directed at the base metal, and the rod is dipped intermittently.
- Furnace brazing: in furnace type, the parts are assembled with filler metal placed in or around the joints that are coated by a flux. Next, the pieces are placed in a furnace set at brazing temperature for 2 or 3 minutes, depending on the thickness. The parts are finally air-cooled to solidify the joints.
- Dip brazing: for the dip method, there are slots or recessed grooves for the filler metal, which prevent it from being washed into the flux bath. The parts are then assembled in a fixture, thoroughly dried, and then immersed in a molten flux bath for 30 to 45 seconds.
Aluminum vs Magnesium Welding And Brazing
Magnesium brazing is similar to aluminum in terms of heat treatment and mechanical properties. Like aluminum, magnesium has a low melting point and conducts heat well. However, magnesium is less dense compared to aluminum, so it is lighter but still strong.
Nonetheless, magnesium can get weaker than the surrounding metal as a byproduct of the heat used during the brazing process. That’s why more caution is required when joining magnesium alloys.

Soldering Magnesium alloys
Soldering is another suitable magnesium joining method that has its ups and downs. Soldering occurs at a somewhat lower temperature, meaning there is little to no risk of defects.
Due to low heat input, soldering is suitable for repairing small surface defects in castings, small dents in sheet metal, and other minor treatments. However, the downsides of low heat soldering don’t perform in stress areas due to low strengths and brittle joints.