Metal plating is the process of applying a thin layer of metal coating on the metal surface, substrate, component, part, or product. The plating process achieves numerous benefits by combining the advantages of two different materials.
For example, steel has positive mechanical characteristics like high strength, and it’s relatively cheap. So, by coating steel with zinc, you can achieve corrosion resistance and combine the benefits of both metals.

There are many reasons metal plating is performed, from industrial to pure decorative purposes. This article discusses the types of metal plating, how they are performed, the most commonly used metals for plating material, and the coating characteristics when welding.
Reasons For Metal Plating
- To harden the surface
- To reduce friction
- Improving corrosion resistance
- Improving paint adhesion
- Improving wear resistance
- Alter the metal conductivity
- Increase radiation shielding
- For decorative purposes like jewelry
- Improving magnetism
- Increasing strength
- Enhancing solderability
Metal Plating Steps In Short
There are a few very different metal plating processes, which we will explain in more detail below. But, most follow a similar steps pattern:
- Cleaning and Surface Preparation of Metal:
- Oxide removal
- Electro-polishing
- Alkaline cleaning
- Metal Plating (see below for description):
- Electroless plating (autocatalytic)
- Electroplating
- Immersion plating
- Carburizing
- Physical Vapor Deposition (PVD)
- Finishing and Protection Treatments:
- Phosphating
- Chromate conversion
- Anodizing
Types Of Metal Plating
The electroplating process is most commonly used. But, each of the plating methods has its advantages. So, let’s explain their fundamentals before proceeding with the individual metal coatings and their welding characteristics.
Electroplating
The electroplating process involves using electricity to instigate a chemical reaction between the plated metal and the plating metal.
The two metals are placed in an electrolyte solution as electrodes.
The cathode (the object receiving the coating) and the anode (the metal used as a coating material). The electricity flows through the circuit, and the electrolyte solution splits atoms from the coating metal (anode).
The negative cathode receives this small amount of metal released into the electrolyte solution, and the plating process is completed.
Numerous metals are suitable for electroplating, including tin, zinc, gold, silver, copper, chrome, nickel, platinum, lead, and rhodium plating.
Electroless Plating
The electroless plating process plates the base metal by a chemical reaction without the need for electricity. It’s less costly and simpler to perform than the electroplating process.
The electroless reaction occurs when hydrogen is released by a reducing agent and gets oxidized, producing a negative charge on the part’s surface.
The most significant advantage of the electroless process is that it allows a nonconductive material to be electroplated. Additionally, it plates the surface evenly, even if the part has irregular shapes, holes, and crevices.
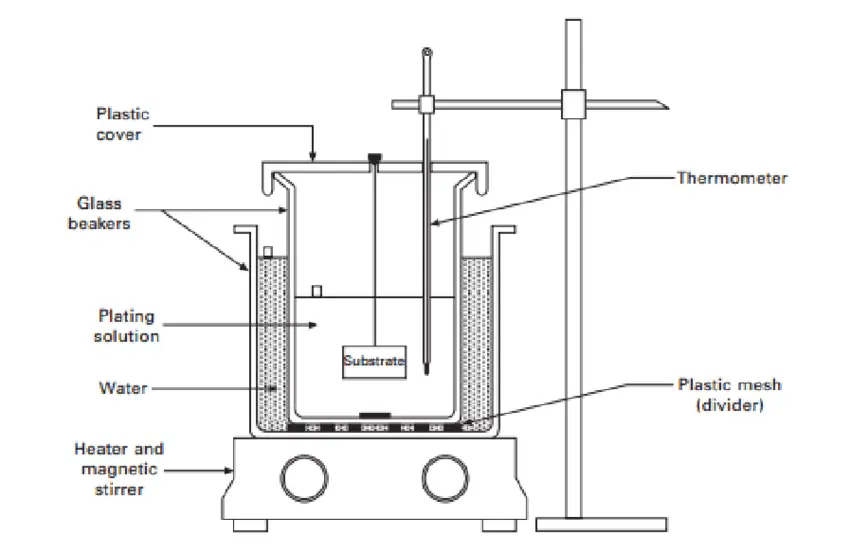
Immersion Plating
The immersion plating process works by submerging the plated part into a solution of noble metal ions.
This adheres the noble metal layers to another metal’s surface and improves plated metal characteristics.
This process is also referred to as replacement or dip plating. It doesn’t require an electrical current because it’s a pure chemical process.
This type of plating is used to improve electrical resistance, appearance, torque tolerance, wear, and corrosion resistance, and sometimes to enhance electrical conductivity.
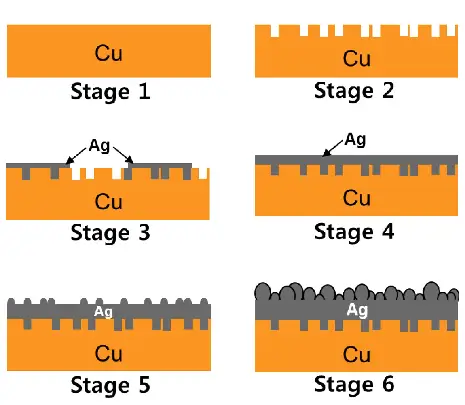
Carburizing
Carburizing is a heat treatment process that produces a wear-resistant surface of the treated part. But, it doesn’t jeopardize the strength of the inner base metal. This is why it’s also referred to as case hardening. After machining, it is usually used with low carbon steel to improve its hardness.
The part is heated in a sealed atmosphere furnace or a pit furnace; then, the carburizing gases are released, affecting the carbon diffusion depth. After, the treaded element is either chilled slowly or quenched directly in oil.
Physical Vapor Deposition (PVD)
Physical vapor deposition is a process of depositing thin films on the plated metal. The solid coating material of metals like aluminum, chromium, and titanium evaporates from heating or ion bombarding. The nitrogen gas is paramount to PVD; it forms a compound with the vapor deposits the plating metal to the part.
The PVD provides a very thin layer on the plated metal’s surface and a powerful bond between the two. This process is used in aerospace, medical, optics, firearms, and automotive industries because of its strong corrosion resisting properties, good impact strength, and high-temperature resistance.
Common Metal Platings
Depending on the chosen metal for plating, you will achieve different results. Additionally, when welding or otherwise treating the plated metal, the coating layer plays a significant role in how to treat the metal. That’s why it’s almost always better to weld the metal before plating it.
Zinc Plating
Carburizing is a heat treatment process that produces a wear-resistant surface of the treated part. But, it doesn’t jeopardize the strength of the inner base metal. This is why it’s also referred to as case hardening.
After machining, it is usually used with low carbon steel to improve its hardness. To achieve corrosion resistance with the most common steel, zinc plating is often applied. The layer of zinc hydroxide uses carbon dioxide to form a dull gray layer of zinc carbonate on plated metal through a chemical reaction.

Zinc plating is a versatile coating material used in numerous applications. For example, the auto and construction industry largely depends on zinc plating to prevent oxidation and corrosion. The zinc plating process also protects car body parts.
Another term for zinc plated steel is galvanized steel. This is a hazardous metal to weld because it releases incredibly toxic fumes. So, any welding should is ideally done before galvanization, not after. If you must weld zinc plated steel, it is necessary to use professional-grade protective equipment like PAPR systems and weld in a highly ventilated area.
Cadmium Plating
Cadmium plating is often applied to steel, cast iron, and copper in industries like transportation, aerospace, and electronics. This layer provides excellent conductivity and paints adhesion ability. Additionally, it offers high resistance in saltwater, so it’s used in the marine industry.
But, it does corrode in other environments. So, sometimes it’s necessary to apply a chromate layer above it. Unfortunately, cadmium is a very toxic element. You should never inhale it because cadmium poisoning leads to pneumonitis and pulmonary edema.

But, even if the poisoning is mild, cadmium will linger for a long time in your body. It accumulates in the bones and damages the blood and kidneys. So, welding cadmium plated steel requires PAPR PPE and a highly ventilated environment as well. Cadmium also wreaks havoc on the environment if released in water. So, this metal has a limited application today.
Tin Plating
Tin plating is used when the plated metal must be non-toxic, resistant to corrosion, and ductile. That’s why tin plating is prevalent in the food processing industry.
Most food cans and cooking utensils are tin-plated for corrosion resistance, and tin is a safe, non-toxic metal.
Welding tin-coated metal is difficult because tin melts at a very low temperature of just 232C. So, this layer should be removed before welding.

Nickel Plating
Nickel plating can be a primary plating metal or serve as an underlayer for silver and gold plating to prevent substrate migration.
Nickel provides strong anti-corrosive characteristics and offers a good-looking finish appearance. It is applied to metals like copper, brass, zinc, steel, and aluminum. Additionally, it’s possible to do nickel plating on plastics to increase part’s lifespan.
Welding nickel-plated steel, or other metals, is very challenging. First, it’s very common for nickel plating to be done over a copper-plated layer.

So, you most likely have two layers to worry about. The safest road is to grind off any plating, weld, and re-plate as necessary.
Chrome Plating
Chrome plating can be divided into two categories; hard chrome also referred to as industrial chrome and decorative chrome plating.
Hard chrome plating lacks the brilliance and luster of decorative chrome, but it serves a practical purpose. This layer is added to mechanical parts for heavy machines. The decorative chromium plating has a higher shine, and it’s often done for looks. It is used in architectural details, interiors, antique cars, motorcycle parts, etc.

Like nickel plating, chromium plating is done over a thin layer of copper because chromium has tiny pores, leading to base metal corrosion and flaking. Welding chromium-plated metal releases toxic fumes. So, you should grind off the chrome and copper coating before welding the part.
Copper Plating
Copper plating is used as a primary coating layer or an underlying layer, as we discussed with chrome and nickel plating examples.
Electronic components like circuit boards and connectors are often copper plated.
This plating method improves the conductivity, anti-corrosion, malleability, and anti-bacterial properties.
An interesting example of copper plating is a typical MIG welding wire. The mild steel wire is copper treated to prevent corrosion.

Silver Plating

Silver plating is applied to brass, copper, and nickel to improve thermal and electrical conductivity. Plus, silver has a low melting point and weight and good lubricity. It is often used in the automotive industry but also for electrical components, shims, gaskets, and bearings.
However, silver plating is not without cons. It may experience galvanic corrosion and dissimilar metal corrosion. Plus, prolonged moisture exposure may lead to flaking, leaving the underlying metal vulnerable to corrosion.
Gold Plating

Gold plating has exceptional corrosion-resistant abilities at every temperature, high ductility, and electrical conductivity.
Gold is primarily used to plate electrical connectors in electronic components.
Additionally, gold is applied to create less expensive jewelry, thanks to its looks.
Conclusion
The metal plating field is vast, and this article barely scraped the surface. Every metal plating process we discussed and common industrial plating metals have entire books dedicated to them. But, we briefly explained metal plating from the welder’s point of view.
Remember that every metal part may be plated, and an untrained eye may not notice the added protective barrier. So, ensure that you always use adequate PPE and practice caution. Compounds like hexavalent chromium, zinc, cadmium, lead, and others pose significant health hazards and require abiding by all welding safety codes.
Resources:
- Electroplating 101: How Metal Plating Works at formlabs.com
- Types of Metal Plating at wisconsinmetaltech.com
- Electroless Plating at www.corrosionpedia.com